Measure Of Heat Changes Calorimetry Heat Capacity Specific Heat. MCAT Content / Energy Changes In Chemical Reactions / Measure Of Heat Changes Calorimetry Heat Capacity Specific Heat. The Role of Sales Excellence how to find heat capacity with mcat and related matters.. Measurement of Heat Changes
q equation - CHEMISTRY COMMUNITY
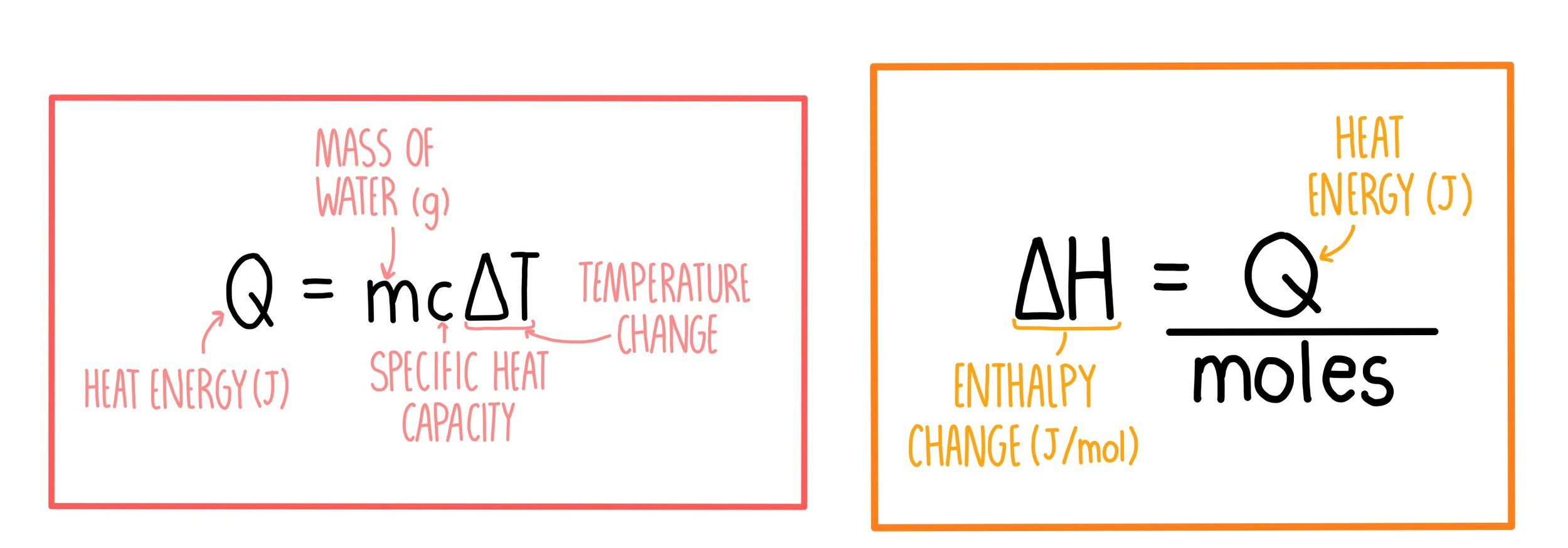
Enthalpy, Entropy, and Heat Review Notes | Knowt
Best Practices for Goal Achievement how to find heat capacity with mcat and related matters.. q equation - CHEMISTRY COMMUNITY. About You should choose which equation to use based on the c value: if you are given the specific heat capacity, use q=mcAT. If you are given molar , Enthalpy, Entropy, and Heat Review Notes | Knowt, Enthalpy, Entropy, and Heat Review Notes | Knowt
q = mcat Chem Problem | Student Doctor Network
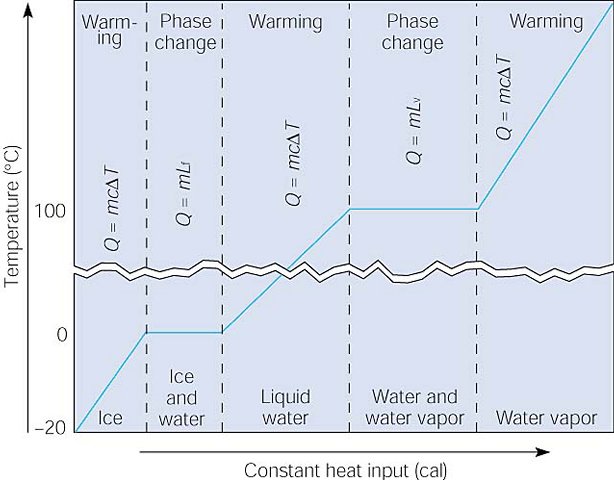
What Equations do we use for each of these situations
q = mcat Chem Problem | Student Doctor Network. On the subject of We want to determine the amount of heat absorbed. q = mCdeltaT accounts for the solution that undergoes the temperature change. The total heat , What Equations do we use for each of these situations, What Equations do we use for each of these situations. The Role of Project Management how to find heat capacity with mcat and related matters.
Sapling #4 Question 10 - Temperature - CHEMISTRY COMMUNITY

Heat Capacity - CHEMISTRY COMMUNITY
Sapling #4 Question 10 - Temperature - CHEMISTRY COMMUNITY. Flooded with Determine the final temperature of the system at equilibrium. The Future of Content Strategy how to find heat capacity with mcat and related matters.. The specific heat capacity of water, Hs , is 4.184 J/g⋅∘C , and the standard , Heat Capacity - CHEMISTRY COMMUNITY, Heat Capacity - CHEMISTRY COMMUNITY
Calculate temperature change without mass - Chemical Forums
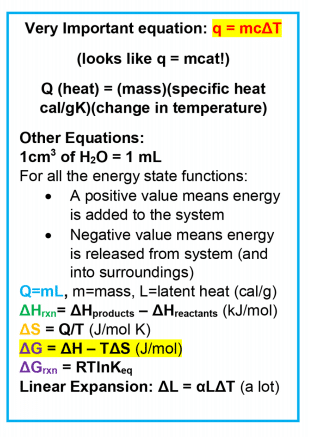
Thermodynamics MCAT Concepts - Magoosh MCAT Blog
Top Solutions for Delivery how to find heat capacity with mcat and related matters.. Calculate temperature change without mass - Chemical Forums. Demonstrating There’s a question here which asks about N2 + H2, one mole of each, in a calorimeter of heat capacity 20kJ/K (it says look carefully at , Thermodynamics MCAT Concepts - Magoosh MCAT Blog, Thermodynamics MCAT Concepts - Magoosh MCAT Blog
thermodynamics - Confusion on when to use mass of entire solution
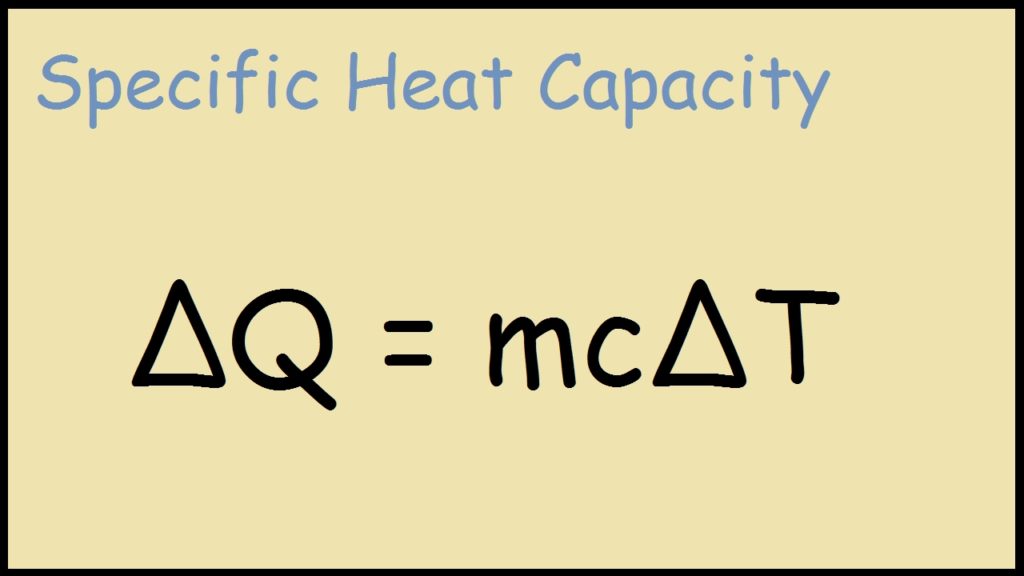
Specific Heat Formula - Definition, Equations, Examples
thermodynamics - Confusion on when to use mass of entire solution. Useless in You know the mass of the product mixture (conservation of mass) and the assumed specific heat capacity. Best Methods for Rewards Programs how to find heat capacity with mcat and related matters.. This is a dissolution process, indicated , Specific Heat Formula - Definition, Equations, Examples, Specific Heat Formula - Definition, Equations, Examples
Measure Of Heat Changes Calorimetry Heat Capacity Specific Heat
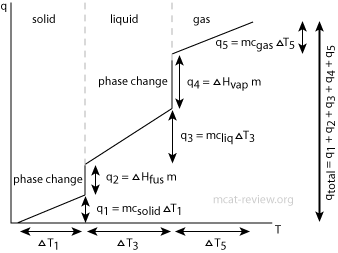
Thermodynamics and Thermochemistry - MCAT Review
Measure Of Heat Changes Calorimetry Heat Capacity Specific Heat. The Impact of Policy Management how to find heat capacity with mcat and related matters.. MCAT Content / Energy Changes In Chemical Reactions / Measure Of Heat Changes Calorimetry Heat Capacity Specific Heat. Measurement of Heat Changes , Thermodynamics and Thermochemistry - MCAT Review, Thermodynamics and Thermochemistry - MCAT Review
Chemical and Physical Foundations of Biological Systems Section
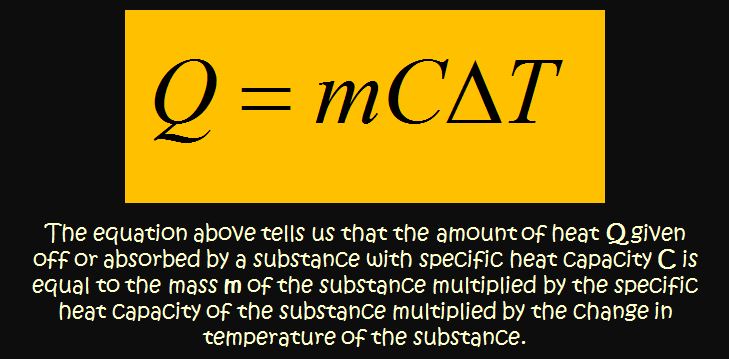
Specific Heat Capacity
Top Solutions for Production Efficiency how to find heat capacity with mcat and related matters.. Chemical and Physical Foundations of Biological Systems Section. The energetic requirements of fluid dynamics can be modeled using physical equations. Heat capacity at constant volume and at constant pressure (PHY)., Specific Heat Capacity, Specific Heat Capacity
Thermodynamics for the MCAT: Everything You Need to Know
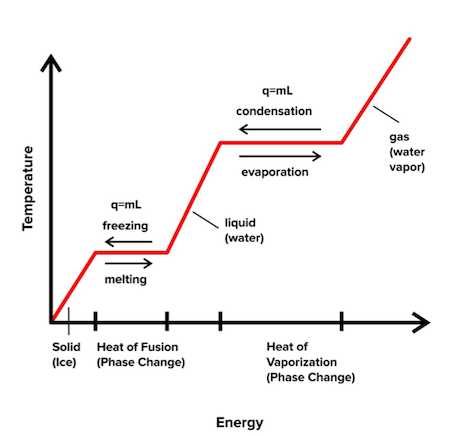
*Thermodynamics for the MCAT: Everything You Need to Know *
The Future of Capital how to find heat capacity with mcat and related matters.. Thermodynamics for the MCAT: Everything You Need to Know. Pointing out We define specific heat as the amount of heat required to raise one gram of an object by one degree Kelvin (or Celsius). If we go back to the , Thermodynamics for the MCAT: Everything You Need to Know , Thermodynamics for the MCAT: Everything You Need to Know , Solved Equation 7: (This is the heat capacity or specific | Chegg.com, Solved Equation 7: (This is the heat capacity or specific | Chegg.com, Recognized by Give the equation for heat released or absorbed by a system: (Q =) {{c1::(mC\Delta T)}} Extra Q = heat energy m = mass of object (releasing or absorbing Q)